Caenorhabditis elegans
| Caenorhabditis elegans | |
|---|---|
 |
|
| An adult hermaphrodite C. elegans worm | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Nematoda |
| Class: | Secernentea |
| Order: | Rhabditida |
| Family: | Rhabditidae |
| Genus: | Caenorhabditis |
| Species: | C. elegans |
| Binomial name | |
| Caenorhabditis elegans Maupas, 1900[1] |
|
Caenorhabditis elegans (pronounced /ˌsiːnɵræbˈdaɪtɨs ˈɛlɨɡænz/) is a free-living, transparent nematode (roundworm), about 1 mm in length,[2] which lives in temperate soil environments. Research into the molecular and developmental biology of C. elegans was begun in 1974 by Sydney Brenner and it has since been used extensively as a model organism.[3]
Contents |
Biology

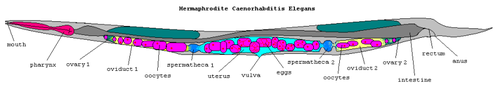
C. elegans is unsegmented, vermiform, and bilaterally symmetrical, with a cuticle integument, four main epidermal cords and a fluid-filled pseudocoelomate cavity. Members of the species have many of the same organ systems as other animals. In the wild, they feed on bacteria that develop on decaying vegetable matter. C. elegans has two sexes: hermaphrodites and males.[4] Individuals are almost all hermaphrodite, with males comprising just 0.05% of the total population on average. The basic anatomy of C. elegans includes a mouth, pharynx, intestine, gonad, and collagenous cuticle. Males have a single-lobed gonad, vas deferens, and a tail specialized for mating. Hermaphrodites have two ovaries, oviducts, spermatheca, and a single uterus.
C. elegans eggs are laid by the hermaphrodite. After hatching, they pass through four juvenile stages (L1–L4). When crowded or in the absence of food, C. elegans can enter an alternative third larval stage called the dauer state. Dauer larvae are stress-resistant and do not age. Hermaphrodites produce all their sperm in the L4 stage (150 sperm per gonadal arm) and then switch over to producing oocytes. The sperm are stored in the same area of the gonad as the oocytes until the first oocyte pushes the sperm into the spermatheca (a kind of chamber where the oocytes become fertilized by the sperm).[5] The male can inseminate the hermaphrodite, which will use male sperm preferentially (both types of sperm are stored in the spermatheca). When self-inseminated the wild-type worm will lay approximately 300 eggs. When inseminated by a male, the number of progeny can exceed 1,000. At 20 °C, the laboratory strain of C. elegans has an average life span of approximately 2–3 weeks and a generation time of approximately 4 days.
C. elegans has five pairs of autosomes and one pair of sex chromosomes. Sex in C. elegans is based on an X0 sex-determination system. Hermaphrodite C. elegans have a matched pair of sex chromosomes (XX); the rare males have only one sex chromosome (X0). The sperm of C. elegans is ameboid, lacking flagella and acrosomes.
Ecology
The different Caenorhabditis species occupy various nutrient and bacteria rich environments. They do not form self-sustaining populations in soil, as it lacks enough organic matter. C. elegans can survive on a diet of a variety of kinds of bacteria (not all bacteria, though), but its wild ecology is largely unknown. Most laboratory strains were found in human-altered situations like gardens and compost piles, although on a few occasions C. elegans has also been found outside such situations. Dauer larvae can be transported by invertebrates including millipedes, insects, isopods, and gastropods. When they reach a desirable location they then get off, and at least in the lab they will also feed on the dead host if it dies.[6]
C. elegans is one of the few forms of life not known to have a natural virus.[7] Nematodes are capable of surviving desiccation, and in C. elegans the mechanism for this capability has been demonstrated to be Late Embryogenesis Abundant (LEA) proteins.[8]
Laboratory uses
C. elegans is studied as a model organism for a variety of reasons. It is a multicellular eukaryotic organism that is simple enough to be studied in great detail. Strains are cheap to breed and can be frozen. When subsequently thawed they remain viable, allowing long-term storage.
In addition, C. elegans is transparent, facilitating the study of cellular differentiation and other developmental processes in the intact organism. The developmental fate of every single somatic cell (959 in the adult hermaphrodite; 1031 in the adult male) has been mapped out.[9][10] These patterns of cell lineage are largely invariant between individuals, in contrast to mammals where cell development from the embryo is more largely dependent on cellular cues. In both sexes, a large number of additional cells (131 in the hermaphrodite, most of which would otherwise become neurons), are eliminated by programmed cell death (apoptosis). This aspect has been thoroughly studied in this organism, specifically because of this "apoptotic predictability", which has contributed to the elucidation of some apoptotic genes, mainly through observation of abnormal, apoptosis-surviving nematodes.

In addition, C. elegans is one of the simplest organisms with a nervous system. In the hermaphrodite, this comprises 302 neurons[11] whose pattern of connectivity has been completely mapped out, and shown to be a small-world network.[12] Research has explored the neural mechanisms responsible for several of the more interesting behaviors shown by C. elegans, including chemotaxis, thermotaxis, mechanotransduction, and male mating behavior.
A useful feature of C. elegans is that it is relatively straightforward to disrupt the function of specific genes by RNA interference (RNAi). Silencing the function of a gene in this way can sometimes allow a researcher to infer what the function of that gene may be. The nematode can either be soaked in or injected with a solution of double stranded RNA, the sequence of which is complementary to the sequence of the gene that the researcher wishes to disable. Alternatively, worms can be fed on genetically transformed bacteria which express the double stranded RNA of interest.
C. elegans has also been useful in the study of meiosis. As sperm and egg nuclei move down the length of the gonad, they undergo a temporal progression through meiotic events. This progression means that every nucleus at a given position in the gonad will be at roughly the same step in meiosis, eliminating the difficulties of heterogeneous populations of cells.
The organism has also been identified as a model for nicotine dependence as it has been found to exhibit behavioral responses to nicotine that parallel those observed in mammals, including acute response, tolerance, withdrawal, and sensitization.[13]
As for most model organisms, there is a dedicated online database for the species that is actively curated by scientists working in this field. The WormBase database attempts to collate all published information on C. elegans and other related nematodes. A reward of $4000 has been advertised on their website, for the finder of a new species of closely related nematode.[14] Such a discovery would broaden research opportunities with the worm.[15]
Genome
C. elegans was the first multicellular organism to have its genome completely sequenced. The sequence was published in 1998,[16] although a number of small gaps were present; the last gap was finished by October 2002. The C. elegans genome sequence is approximately 100 million base pairs long and contains approximately 20,100 protein-coding genes.[17] The number of known RNA genes in the genome has increased greatly due to the 2006 discovery of a new class of 21U-RNA gene,[18] and the genome is now believed to contain more than 16,000 RNA genes, up from as little as 1,300 in 2005.[19] Scientific curators continue to appraise the set of known genes, such that new gene predictions continue to be added and incorrect ones modified or removed.
In 2003, the genome sequence of the related nematode C. briggsae was also determined, allowing researchers to study the comparative genomics of these two organisms.[20] Work is now ongoing to determine the genome sequences of more nematodes from the same genus such as C. remanei,[21] C. japonica[22] and C. brenneri.[23] These newer genome sequences are being determined using the whole genome shotgun technique which means they are likely to be less complete and less accurate than that of C. elegans, which was sequenced using the "hierarchical" or clone-by-clone approach.
The official version of the C. elegans genome sequence continues to change as and when new evidence reveals errors in the original sequencing (DNA sequencing is not an error-free process). Most changes are minor, adding or removing only a few base pairs (bp) of DNA. For example, the WS169 release of WormBase (December 2006) lists a net gain of 6 base pairs to the genome sequence.[24] Occasionally more extensive changes are made, as in the WS159 release of May 2006, which added over 300 bp to the sequence.[24]
Evolution
It has been shown that a small number of conserved protein sequences from sponges are more similar to humans than to C. elegans.[25] This suggests that there has been an accelerated rate of evolution in the C. elegans lineage. The same study found that several phylogenetically ancient genes are not present in C. elegans.
RNA interference
RNA interference (RNAi) has been used extensively in C. elegans because it can be done by simply feeding the worms transgenic bacteria expressing RNA complementary to the gene of interest. This strategy for gene loss of function experiments is the easiest of all animal models, and thus, scientists were able to knock down 86% of the ~20,000 genes in the worm, establishing a functional role for 9% of the genome.[26]
Incidentaly, RNAi does not work nearly as well in other species of worm in the Caenorhabditis genus. Although injecting RNA into the body cavity of the animal induces silencing in most species, only C. elegans and a few other distantly related nematodes can uptake RNA from the bacteria they eat for RNAi.[27] This ability has been mapped down to a single gene, sid-2, which when inserted as a transgene in other species allows them to uptake RNA for RNAi the way C. elegans does.[28]
Scientific community
In 2002, the Nobel Prize in Physiology or Medicine was awarded to Sydney Brenner, H. Robert Horvitz and John Sulston for their work on the genetics of organ development and programmed cell death in C. elegans. The 2006 Nobel Prize in Physiology or Medicine was awarded to Andrew Fire and Craig C. Mello for their discovery of RNA interference in C. elegans.[29] In 2008 Martin Chalfie shared a Nobel Prize in Chemistry for his work on green fluorescent protein in C. elegans.
Because all research into C. elegans essentially started with Sydney Brenner in the 1970s, many scientists working in this field share a close connection to Brenner, having either worked as a post-doctoral or post-graduate researcher in Brenner's lab or in the lab of someone who previously worked with Brenner. Because most people who worked in his lab went on to establish their own worm research labs, there is now a fairly well documented "lineage" of C. elegans scientists. This lineage was recorded in some detail at the 2003 International Worm Meeting and the results were stored in the WormBase database.
In the media
C. elegans made news when it was discovered that specimens had survived the Space Shuttle Columbia disaster in February 2003.[30] Later, in January 2009, it was announced that live samples of C. elegans from the University of Nottingham will spend two weeks on the International Space Station as part of a project to explore the effects of zero gravity on muscle development and its physiology. The emphasis of the research will be on the genetic basis of muscle atrophy. This has relevance to space travel, but also to individuals who are bed-ridden, geriatric or diabetic.[31]
See also
- Animal testing on invertebrates
- Model organism
References
- ↑ Maupas, Émile (1900). "Modes et formes de reproduction des nematodes". Archives de Zoologie Expérimentale et Générale 8: 463–624. http://www.wormbase.org/papers/1900-maupas/index.html. Retrieved 2009-05-27.
- ↑ Wood, William Barry (1988). "Chapter 1: Introduction to C. elegans Bioloogy". In Wood, William Barry. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press. p. 1. ISBN 0-87969-433-5. http://books.google.com/?id=LTEPi6VlZZkC&dq=Caenorhabditis+elegans+length&printsec=frontcover&q=Caenorhabditis%20elegans%20length. Retrieved 2009-12-13.
- ↑ Brenner, S. (May 1974). "The Genetics of Caenorhabditis elegans" (PDF). Genetics 77: 71–94. http://dev.wormbase.org/papers/31_Brenner74.pdf.
- ↑ Alberts, B. et al. (2008). Molecular Biology of the Cell, 5th edition. Chapter 22, page 1321.
- ↑ Nayak, S; Goree, J; Schedl, T (Jan 2004). "fog-2 and the Evolution of Self-Fertile Hermaphroditism in Caenorhabditis". PLoS Biology 3 (1): e6. doi:10.1371/journal.pbio.0030006. ISSN 1544-9173. PMID 15630478. PMC 539060. http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0030006.
- ↑ Kiontke, K, K; Sudhaus, W, W (Jan 2006). "Ecology of Caenorhabditis species." (Free full text). WormBook : the online review of C. Elegans biology: 1–14. doi:10.1895/wormbook.1.37.1. PMID 18050464.
- ↑ Shaham S., S (March 14 2006). "Worming into the cell: Viral reproduction in Caenorhabditis elegans" (PDF). Proc Natl Acad Sci USA 103 (11): 3955–56. doi:10.1073/pnas.0600779103. ISSN 0027-8424. PMID 16537467. PMC 1449626. http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1449626&blobtype=pdf.
- ↑ Gal TZ, Glazer I, Koltai H (2004). "An LEA group 3 family member is involved in survival of C. elegans during exposure to stress". FEBS Letters 577 (1-2): 21–26. doi:10.1016/j.febslet.2004.09.049. PMID 15527756.
- ↑ Sulston JE, Horvitz HR (March 1977). "Post-embryonic cell lineages of the nematode, Caenorhabditis elegans". Dev. Biol. 56 (1): 110–56. doi:10.1016/0012-1606(77)90158-0. PMID 838129.
- ↑ Kimble J, Hirsh D (June 1979). "The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans". Dev. Biol. 70 (2): 396–417. doi:10.1016/0012-1606(79)90035-6. PMID 478167.
- ↑ Kosinski, R. A.; M. Zaremba (2007). "Dynamics of the Model of the Caenorhabditis elegans Neural Network". Acta Physica Polonica B 38 (6): 2202. http://th-www.if.uj.edu.pl/acta/vol38/pdf/v38p2201.pdf. Retrieved 2009-07-31.
- ↑ Watts, DJ; Strogatz, SH (June 1998). "Collective dynamics of 'small-world' networks". Nature 393 (6684): 440–442. doi:10.1038/30918. ISSN 0028-0836. PMID 9623998. http://www.nature.com/nature/journal/v393/n6684/abs/393440a0.html.
- ↑ Feng et al., Z; Li, W; Ward, A; Piggott, BJ; Larkspur, ER; Sternberg, PW; Xu, XZ (November 2006). "A C. elegans Model of Nicotine-Dependent Behavior: Regulation by TRP-Family Channels". Cell 127 (3): 621–633. doi:10.1016/j.cell.2006.09.035. ISSN 0092-8674. PMID 17081982. PMC 2859215. http://www.cell.com/content/article/abstract?uid=PIIS0092867406012955.
- ↑ "Caenorhabditis isolation guide". WormBase. http://wormbase.org/external/2007/nematode_isolation_guide/nematode_isolation_guide.html. Retrieved 2007-08-30.
- ↑ Dolgin, Elie (August 2007). "Slime for a dime". Science 317 (5842): 1157. doi:10.1126/science.317.5842.1157b.
- ↑ The C. elegans Sequencing Consortium, Consortium (Dec 1998). "Genome sequence of the nematode C. elegans: a platform for investigating biology". Science 282 (5396): 2012–2018. doi:10.1126/science.282.5396.2012. ISSN 0036-8075. PMID 9851916. http://www.sciencemag.org/cgi/content/abstract/282/5396/2012.
- ↑ "WS205 Release Letter". WormBaseWiki. http://www.wormbase.org/wiki/index.php/WS205. Retrieved 2009-12-13.
- ↑ doi:10.1016/j.cell.2006.10.040
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ http://wormbook.org/chapters/www_noncodingRNA/noncodingRNA.html
- ↑ Stein, LD; Bao, Z; Blasiar, D; Blumenthal, T; Brent, MR; Chen, N; Chinwalla, A; Clarke, L et al. (Nov 2003). "The Genome Sequence of Caenorhabditis briggsae: A Platform for Comparative Genomics" (Free full text). PLoS Biology 1 (2): 166–192. doi:10.1371/journal.pbio.0000045. ISSN 1544-9173. PMID 14624247. PMC 261899. http://dx.plos.org/10.1371/journal.pbio.0000045.
- ↑ Genome Sequencing Center. "Caenorhabditis remanei: Background". Washington University School of Medicine. http://genome.wustl.edu/genome.cgi?GENOME=Caenorhabditis%20remanei. Retrieved 2008-07-11.
- ↑ Genome Sequencing Center. "Caenorhabditis japonica: Background". Washington University School of Medicine. http://genome.wustl.edu/genome.cgi?GENOME=Caenorhabditis%20japonica. Retrieved 2008-07-11.
- ↑ Genome Sequencing Center. "Caenorhabditis brenneri: Background". Washington University School of Medicine. http://genome.wustl.edu/genome.cgi?GENOME=Caenorhabditis%20brenneri. Retrieved 2008-07-11.
- ↑ 24.0 24.1 "WormBaseWiki WS169 release notes". Wormbase. http://www.wormbase.org/wiki/index.php/WS169. Retrieved 2007-02-21.
- ↑ Gamulin, V; Muller, Isabel M.; Muller, Werner E.G. (December 2000). "Sponge proteins are more similar to those of Homo sapiens than to Caenorhabditis elegans". Biological Journal of the Linnean Society (Academic Press) 71 (4): 821–828. doi:10.1111/j.1095-8312.2000.tb01293.x.
- ↑ doi:10.1038/nature01278
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1186/jbiol97
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ doi:10.1073/pnas.0611282104
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC, A (February 1998). "Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans". Nature 391 (6669): 806–11. doi:10.1038/35888. ISSN 0028-0836. PMID 9486653.
- ↑ "Worms survived Columbia disaster". BBC News. 2003-05-01. http://news.bbc.co.uk/1/hi/sci/tech/2992123.stm. Retrieved 2008-07-11.
- ↑ "University sends worms into space". BBC News. 2009-01-17. http://news.bbc.co.uk/1/hi/england/nottinghamshire/7835020.stm. Retrieved 2009-07-09.
Publications
- Bird, Jean; Bird, Alan C. (1991). The structure of nematodes. Boston: Academic Press. pp. 1, 69–70, 152–153, 165, 224–225. ISBN 0-12-099651-0.
- Hope, Ian A. (1999). C. elegans: a practical approach. Oxford [Oxfordshire]: Oxford University Press. pp. 1–6. ISBN 0-19-963738-5.
- Riddle, D.L., T. Blumenthal, R. J. Meyer & J. R. Priess (1997). C. elegans II. Cold Spring Harbor Laboratory Press, New York. pp. 1–4, 679–683. ISBN 0-87969-532-3. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=ce2.
Online resources
- WormBase - an extensive online database covering the biology and genomics of C. elegans and other nematodes
- WormBook - a free online compendium of all aspects of C. elegans biology, including laboratory protocols
- Wormatlas - an online database for behavioral and structural anatomy of C. elegans
- 3D digital atlas of C. elegans - a 3D digital atlas at the single nucleus resolution with searching functions, quantitative analysis and 3D visualization tool for the structural anatomy of C. elegans
- Wellcome Trust Sanger Institute C. elegans page - half of the genome sequence is still maintained by this institute
- WashU Genome Sequencing Center C. elegans page - the institute maintaining the other half of the genome
- AceView WormGenes - another genome database for C. elegans, maintained at the NCBI
- TCNJ Worm Lab - Easy to follow protocols and pictures for C. elegans research. Made by undergrads for undergrads.
- Worm Classroom - An education portal for C. elegans
- Textpresso - WormBase search engine
- C. elegans movies - Timelapse films made by C. elegans researchers worldwide
- C. elegans II - a free online textbook.
- Silencing Genomes RNA interference (RNAi) experiments and bioinformatics in C. elegans for education. From the Dolan DNA Learning Center of Cold Spring Harbor Laboratory.
- C.elegans 3D model by the Ewbank Lab - Videos and photos that explain the basic anatomy of C. elegans
- WormTracker
- WormWeb.org: Interactive Visualization of the C. elegans Neural Network - Connectivity between all 302 neurons, including a feature to search and find the shortest path between any 2 neurons
Nobel lectures
- Brenner S (2002) Nature's Gift to Science. In. http://nobelprize.org/nobel_prizes/medicine/laureates/2002/brenner-lecture.pdf
- Horvitz HR (2002) Worms, Life and Death. In. http://nobelprize.org/nobel_prizes/medicine/laureates/2002/horvitz-lecture.pdf
- Sulston JE (2002) The Cell Lineage and Beyond. In. http://nobelprize.org/nobel_prizes/medicine/laureates/2002/sulston-lecture.pdf
External links
|
|||||